
Đang tải...

Đang tải...
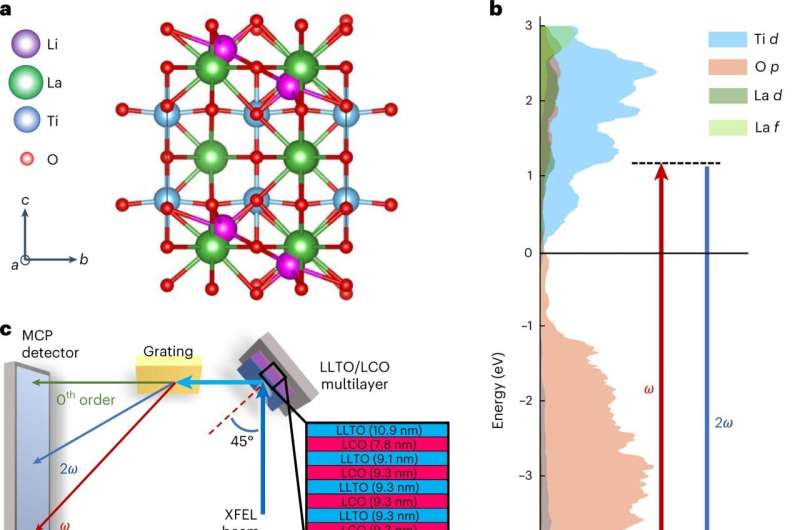
LLTO structure and experimental geometry. a, Basic crystal structure of LLTO, consisting of alternating Li-rich and poor layers and Ti and O octahedra. b, Calculated Partial Density of States (DOS) for LLTO and transitions change specified for the XUV-SHG probe. c, Overview of experimental setup used to measure XUV-SHG data in reflectance geometry. The inset shows the layered sample structure with repeated LLTO and LCO layers. d, Schematic representation of the LCO–LLTO stack forming the prototype battery, with the XUV-SHG process indicated on the top surface; note that LLTO is polycrystalline in the measured film despite the schematic representation. XFEL, X-ray free electron laser. MCP, microchannel plate.
An international team of researchers, including nanoengineers at the University of California San Diego, have discovered nanoscale changes inside solid-state batteries that could yield new insights into improving battery performance.
Using computer simulations and X-ray experiments, researchers were able to "see" in detail why lithium ions move slowly in solid electrolytes —specifically at the electrode-electrolyte interface . Their studies revealed that faster vibrations at the interface make it harder for lithium ions to move there than the rest of the material. Their findings, published April 27 in the Journal of Nature Materials , could lead to new strategies for enhancing ionic conductivity in solid-state batteries.
Solid-state batteries, containing electrolytes made of solid materials, promise to be safer, as well as last longer and more efficient than traditional lithium-ion batteries with flammable liquid electrolytes.
But a big problem with these batteries is that the movement of lithium ions is more limited, especially when the electrolyte is in contact with the electrode.
"Our ability to make a better solid-state battery is hampered by the fact that we don't know exactly what is happening at the interface between these two solids," said study co-author Tod Pascal, professor of nanoengineering and chemical engineering, said. Fellow of the Center for Sustainable Energy and Energy at UC San Diego Jacobs School of Engineering. "This work provides a new microscope for looking at these types of interfaces. By seeing what the lithium ions are doing and understanding how they move through the battery, we can begin engineering ways to get them back and forth more efficiently."
For this study, Pascal teamed up with his longtime collaborator, Michael Zuerch, a professor of chemistry at UC Berkeley, to develop a technique for directly probing lithium ions at the interface. Over the past three years, the two groups have been working on developing an entirely new spectroscopic method for probing buried functional interfaces, such as those found in batteries. Pascal's lab led the theoretical work, while Zuerch's lab led the experimental work.
The new technique they developed combines two established approaches. The first is X-ray absorption spectroscopy, which involves irradiating a material with an X-ray beam to determine its atomic structure. This method is useful for probing lithium ions deep within the material, but not at the exposed surface. So the researchers used a second method, called second harmonic generation, that can identify specific atoms at an interface.
It involves colliding atoms with two consecutive pulses of high-energy particles—in this case, intense beams of X-rays at a specified energy—so that the electrons can reach a state. high energy state, known as the double excited state. This excited state is not long-lived, which means that the electrons eventually return to the ground state and release the absorbed energy, which is then detected as a signal. The key here is that only certain atoms, such as those at the interface, can undergo this double excitation. Therefore, the signals detected from these experiments will necessarily and only provide information about what is happening right at the interface, Pascal explained.
The researchers used this technique on a solid-state battery model consisting of two commonly used battery materials: titanium lanthanum lithium oxide as the solid electrolyte and lithium cobalt oxide as the cathode.
To verify that the signals they saw were indeed coming from the interface, the researchers performed a series of computer simulations, based on methods developed in Pascal's research group. When the researchers compared experimental and computational data, they found that the signals matched almost exactly.
"The theoretical work allows us to fill in the blanks and provides clarity about the signals we see in the experiments," said the study's first co-author, Sasawat Jamnuch, PhD in nanoengineering said. students in Pascal's research group have just defended their doctoral thesis. "But a bigger advantage of the theory is that we can use it to answer additional questions. For example, why do these signals appear the way they are?"
Unlock ionic movement at the interface
Jamnuch and Pascal have gone a step further. They modeled the dynamics of lithium ions in a solid electrolyte and made an unexpected discovery. They found that high-frequency vibrations were occurring at the electrolyte interface, and that these vibrations further restricted the movement of the lithium ions compared to vibrations in the rest of the material.
“This is one of the main findings of this study that we were able to extract theoretically,” says Jamnuch. Battery researchers have long suspected that the incompatibility between the solid electrolyte and the electrode material limits the movement of lithium ions at the interface. Now, Jamnuch, Pascal and colleagues show that something else is also going on.
“There is actually some intrinsic resistance to ion movement in this material right at the interface,” says Pascal. "The barrier to the passage of lithium ions is not only a function of two solid materials mechanically incompatible with each other, it is also a function of vibrations within the material itself."
He describes the barrier to ion movement as similar to what a ball would experience if it bounced inside a room where the walls are also moving.
"Imagine a room with a ball in the back, and the ball is trying to move forward," he said. "Now imagine that the edges of the room are also moving back and forth, causing the ball to bounce from side to side. Total energy is conserved, so if the ball bounces more from side to side. the other, it has to move less back to front In other words, the faster the sides move, the more time the ball bounces around and the longer it takes to move forward.
"Similarly, in these solid-state batteries , the path that lithium ions take through the material is influenced by the fact that the material itself is oscillating at a higher frequency at the interface surface than at the interface surface. So, even if there is perfect compatibility between the electrolyte and the electrode material, there will still be resistance to lithium diffusion across the interface due to high frequency vibrations. This."
Thanks to their computational work, the researchers laid the groundwork for future solid-state battery designs.
“One idea is to slow down the vibrations at the interface of the solid electrolyte material,” says Jamnuch. "You can do that by doping the interface with heavy elements, for example."
“Now that we understand more about how lithium ions pass through this system, we can rationally design new systems that make it easier for ions to pass,” says Pascal. "We found new knobs to rotate, new ways to optimize these systems."